Abstract
Background
Autologous CD30-directed chimeric antigen receptor T-cell (CD30.CAR-T) therapy is well tolerated, with significant clinical efficacy in relapsed or refractory (r/r) classical Hodgkin Lymphoma (cHL) patients, as demonstrated in phase 1/2 clinical studies [LCCC 1532-ATL (NCT02690545) and H-37966 (RELY-30, NCT02917083); Ramos et al., 2020] and the pilot part of CHARIOT phase 2 trial (TESSCAR001, NCT0535288; Ahmed et al., 2021).
cHL is characterized by the presence of malignant Hodgkin Reed-Sternberg (HRS) cells, which frequently harbor amplifications in chromosome 9p24.1, leading to overexpression of programmed death-ligand 1 (PD-L1) (Chen et al., 2017). The efficacy and safety profile of programmed death (PD)-1 checkpoint inhibitors, nivolumab and pembrolizumab, in cHL have been well demonstrated (Chen et al., 2017; Kuruvilla et al., 2021; Younes et al., 2016), with FDA approval for r/r cHL. PD-1 checkpoint inhibitors, in combination with CD30-directed antibody therapy (brentuximab vedotin) or other chemotherapies, have also shown high efficacy in this setting (Advani et al., 2021; Mei et al., 2022; Moskowitz et al., 2021). Preclinical studies demonstrated potential synergism between PD-1 checkpoint inhibitors and CAR-T cell therapy, where PD-1 or PD-L1 antibodies boost CAR-T cell therapy and promote increased tumor cell death in vivo (Cherkassky et al., 2016; Gargett et al., 2016; Moon et al., 2014).
Despite multiple treatment options available in the second line therapy for cHL, 10-40% of patients do not achieve response to salvage chemotherapy (von Keudell and Younes, 2019). Additionally, salvage chemotherapies are associated with short-term toxicity, long-term morbidity and non-lymphoma-related mortality (de Vries et al., 2021; Dores et al., 2020). We have designed a phase 1b/2 trial (ACTION) to evaluate the safety and antitumor activity of CD30.CAR-T cells combined with nivolumab in advanced cHL patients in whom standard frontline chemotherapy has failed.
Study Design and Methods
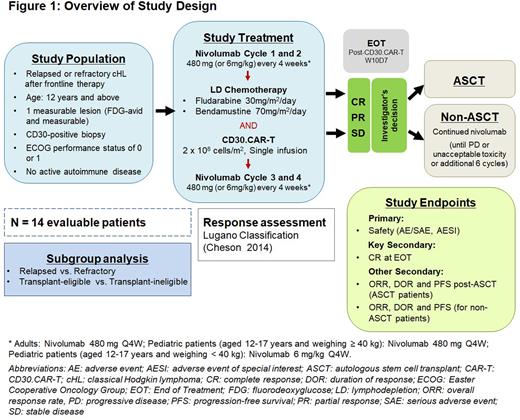
Approximately 15 adult and pediatric patients (≥12 years old) with r/r cHL following progressive disease after standard frontline therapy will be enrolled in the present single-arm, phase 1b/2, multicenter study (ClinicalTrials.gov NCT05352828). Following a leukapheresis procedure for manufacture of CD30.CAR-T cells, patients will be treated with 4 cycles of nivolumab every 4 weeks (Q4W), and a single infusion of CD30.CAR-T cells (given between nivolumab Cycles 2 and 3, and after lymphodepletion with bendamustine and fludarabine). At the end of treatment (EOT) visit (4 weeks after nivolumab Cycle 4) and at the Investigators' discretion, patients with complete response (CR), partial response (PR), or stable disease (SD) will undergo either standard treatment with autologous stem cell transplant (ASCT group) or continue nivolumab Q4W for up to 6 additional cycles, unless patients experience either progressive disease (PD) or unacceptable toxicity, whichever occurs earlier (non-ASCT group). Patients will be followed with periodic response assessments and safety monitoring until end of study (EOS), approximately 3 years after leukapheresis. Long-term follow-up will continue for up to 15 years.
The primary objective of this study is to assess the safety of autologous CD30.CAR-T in combination with nivolumab. The key secondary objective is to evaluate potential anti-tumor activity, as assessed by the CR rate of autologous CD30.CAR-T in combination with nivolumab at EOT in 14 evaluable patients, as per Lugano Classification Revised Response System for malignant lymphoma (Cheson et al., 2014). Other secondary objectives are to assess the overall response rate (ORR), duration of response (DOR), and progression-free survival (PFS) of patients in the ASCT and non-ASCT groups.
This study is ongoing and one patient has been enrolled to date.
Disclosures
Mei:Morphosys: Research Funding, Speakers Bureau; Celgene: Research Funding; Incyte: Research Funding; Novartis: Consultancy; EUSA: Honoraria; Beigene: Research Funding; CTI: Honoraria. Ahmed:Xencor: Research Funding; Chimagen: Consultancy, Research Funding; Tessa Therapeutics: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Seagen: Research Funding; Merck: Research Funding. Beitinjaneh:Kite/Gilead: Membership on an entity's Board of Directors or advisory committees. Grover:ADC: Other: Advisory Board; Kite: Other: Advisory Board; Novartis: Consultancy; Tessa Therapeutics: Consultancy; Genentech: Research Funding. Ramos:Tessa Therapeutics: Consultancy, Patents & Royalties: HPV-specific T cell manufacture, Research Funding; Athenex Therapeutics: Consultancy, Research Funding; Genentech: Consultancy; Novartis: Consultancy; CRISPR Therapeutics: Consultancy. Kang:Tessa Therapeutics: Current Employment. Nadler:Iksuda Therapeutics Ltd: Consultancy; Tessa Therapeutics: Consultancy; Symphogen: Consultancy. Myo:Tessa Therapeutics: Current Employment. Horak:Tessa Therapeutics: Current Employment. Heslop:Fresh Wind Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Ankarys: Membership on an entity's Board of Directors or advisory committees; Immunai: Membership on an entity's Board of Directors or advisory committees; Gilead Biosciences: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kuur Therapeutics: Research Funding; Allovir: Current equity holder in publicly-traded company; Marker Therapeutics: Current equity holder in publicly-traded company; Kiadis: Divested equity in a private or publicly-traded company in the past 24 months; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding.
OffLabel Disclosure:
Nivolumab: Has been approved for adult patients with classical Hodgkin lymphoma that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and brentuximab vedotin, or 3 or more lines of systemic therapy that includes autologous HSCT. This study will investigate the usage of nivolumab in combination with CD30.CAR-T cells for relapsed or refractory classical Hodgkin Lymphoma after failure of frontline therapy.
Author notes
Asterisk with author names denotes non-ASH members.